Red rust and drug quality and safety - a new red rust prevention and removal technology
This article mainly introduces the characteristics and hazards of red rust phenomenon in the pharmaceutical industry, and proposes specific solutions based on the requirements of the 2010 edition of the Chinese Pharmacopoeia and the 2010 edition of GMP; At the same time, the excellent role of HS-210 reagent in red rust removal and passivation was introduced; Finally, the maintenance measures for the pharmaceutical water system after the occurrence of red rust were emphasized.
As is well known, one of the main reasons for using stainless steel in pharmaceutical engineering is its superior corrosion resistance. When reddish brown rust spots appear on the surface of stainless steel, people often think: "Stainless steel does not rust, and if it rusts, it is not stainless steel anymore. It must be a problem with the steel. In fact, this is a one-sided and erroneous view of stainless steel. In fact, stainless steel can also rust under specific conditions, which is called the "red rust phenomenon" in engineering, as shown in Figure 1.
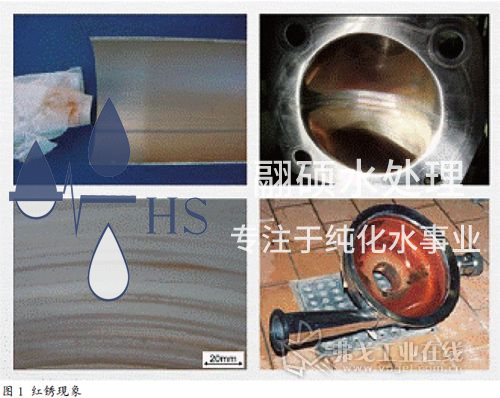
Red rust is a common engineering phenomenon of stainless steel materials in pharmaceutical fluid process systems. Red rust often occurs on the inner walls of the conveying pump chamber, diaphragm valve body and diaphragm, tank body, spray ball inner wall, stainless steel welds and heat affected zones in pharmaceutical water and other fluid process systems. When wiping the stainless steel inner wall with a white cloth or filtering and sampling at the outlet of the water point, yellow or red solid particles are often seen, which are mainly composed of red rust of iron oxide.
There are many factors that can cause red rust, such as high temperature or high pressure environments; Highly corrosive environments such as chlorides; Non stainless steel components; Improper surface preparation (such as welding quality issues, material surface defects, improper cleaning or passivation, etc.) can also induce the formation of red rust.
The harm caused by red rust is significant. It belongs to particulate matter pollution and can affect the quality of pharmaceutical water and the clarity of drugs; Increase the effective workload of the filter; Affects the pressure resistance and corrosion resistance of stainless steel systems; May cause physiological reactions with the final product.
Formation mechanism and classification of red rust
Oxidation is a common form of electrochemical reaction, and its main principle is that one element releases electrons while another element absorbs electrons, forming an oxidation-reduction reaction. In this process, oxygen combines with a certain element in a metal or alloy to form a metal oxide. The basic characteristic of stainless steel corrosion resistance is that the Cr element in the alloy can form a stable chromium rich oxide film on its surface when in contact with oxygen. It is formed instantly in the presence of oxidizing gas in stainless steel. After the formation of the passivation layer, the corrosion resistance of the metal can be improved, and the metal exhibits a unique "inertness", and its oxidation rate will be reduced to a negligible range.
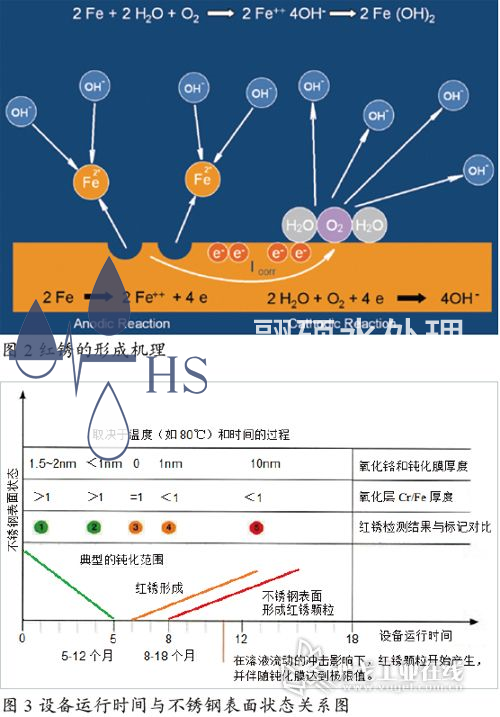
Corrosion is a chemical or electrochemical reaction between a metal and its environment, which can cause unexpected changes in the metal's properties. These reactions can lead to a decrease in the metal's corrosion resistance. Common types of corrosion include uniform corrosion, electrochemical corrosion, crevice corrosion, pitting corrosion, stress corrosion cracking, and intergranular corrosion. Once the passivation layer of stainless steel is damaged for some reason, the oxygen in the water will slowly react with the Fe element released from the metal to form loose iron oxide, and the metal surface will exhibit rust corrosion, commonly known as "red rust". Figure 2 is a simulated mechanism of red rust formation. Although this mechanism is still controversial, it vividly illustrates the chemical process of red rust formation.
Water is an extremely weak electrolyte. At 25 ℃, the ion product constant Kw of water is 1 × 10-14. At 100 ℃, the ion product constant Kw of water is 55 × 10-14. The concentration of [H+] and [OH -] in high-temperature injection water is much higher than that in room temperature purified water systems, resulting in an increase in the rate of chemical reaction between free iron ions and hydroxide ions in the water, ultimately generating iron oxide and causing red rust in the system. Therefore, the system is more prone to red rust when operating under high temperature conditions. Figure 3 shows the relationship between the operating time of the 80 ℃ injection water storage and distribution system and the surface condition of the stainless steel.
According to the degree of occurrence, red rust can be classified into three types: Type I, Type II, and Type III (Figure 4). Type I red rust, also known as migratory red rust, contains oxides and hydroxides derived from various source metals, with Fe2O3 as the main component and a small amount of FeO and Fe (OH) 2. Type I red rust is in the form of particles, loosely attached to the surface of stainless steel, and presents an orange or orange red color, with a tendency to migrate downstream from the point of red rust formation. Type I red rust has the characteristics of easy generation, easy removal, and easy recurrence. Type II red rust belongs to active corrosion formed locally on metal surfaces, mainly composed of Fe2O3, presenting a series of chromatograms from red, orange, blue, purple, gray to black. Type II red rust adheres tightly to the surface of stainless steel and is difficult to remove once formed. It often appears in various forms such as corrosion pits and corrosion cracks, and is related to the corrosion of chlorides or other halides.
Type III red rust is a black oxide produced after heating oxidation, which often occurs on the surface of high-temperature environments (such as pure steam systems). Its main component is Fe3O4. As the red rust layer thickens, the system color changes from gold to blue, and then to varying shades of black. This surface oxidation begins in a stable film form and is almost non granular. Its crystal structure is similar to that of extremely stable magnetite.

Red rust removal reagents and case studies
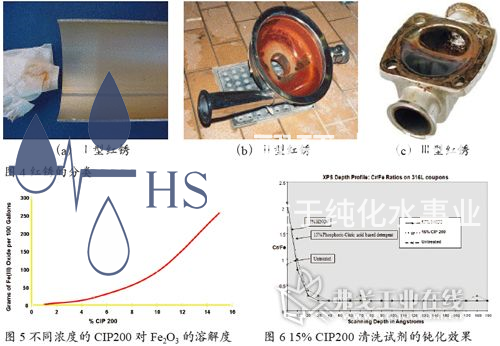
HS-220 and HS-210 reagents are cleaning agents developed by STERIS specifically for the removal of "red rust" and passivation of stainless steel materials. HS-220 is an alkaline cleaning agent mainly used to remove oil stains, protein impurities, and biofilms on the surface of stainless steel, providing a prerequisite for the rust removal and cleaning effect of HS-210; HS-210 is an acidic cleaning agent, especially suitable for special requirements in the pharmaceutical, cosmetics, medical devices, food, and beverage industries. Its main ingredients include phosphoric acid, citric acid, surfactants, and dispersants, which can effectively remove red rust on stainless steel surfaces, especially type I and type II red rust. Figure 5 shows a comparison table of the solubility of Fe2O3 by HS-210 at different concentrations. The solubility of Fe2O3 by 15% CIP can reach 260g/100 gallons.
The main component of CIP200 cleaning reagent is citric acid, which effectively avoids the strong acid corrosion reaction on stainless steel when using strong acids such as nitric acid for cleaning according to convention; At the same time, HS-210 cleaning reagent contains stable surfactants and dispersants, which not only increase surface wetting effect but also reduce the re deposition of dirt; HS-210 cleaning agent can be compatible with a variety of materials, and is in a low foam state at any temperature, which effectively prevents cavitation of centrifugal pump. In addition, it can also be fully rinsed, with very low residue. Of particular importance is that the 15% CIP200 cleaning reagent has the same passivation effect as the 17% HNO3 (Figure 6), which can achieve one-step removal of red rust and pickling passivation in stainless steel systems, greatly simplifying the cleaning process and saving cleaning time. Therefore, the HS-210 cleaning reagent has been widely used for rust removal and passivation in purified water and injection water systems, pure steam systems, liquid preparation systems, and CIP/SIP systems.
Figure 7 shows a case study of a high-temperature injection water storage and distribution system used by the author to remove red rust. The case used 15% CIP200 to achieve one-time removal of red rust and passivation treatment at a high temperature of 80 ℃. Through regular sampling and testing, the rust removal and passivation effects were good.
Summary
To reduce the risk of red rust in pharmaceutical fluid process systems, enterprises need to adopt the management concept of "quality comes from design" and effectively control from the design source. In engineering, the following measures have certain reference value for preventing and controlling the occurrence of red rust:
Reduce the circulating temperature of the injection water system appropriately, such as keeping the system temperature between 70 ℃ and 85 ℃ for circulation;
Strictly follow the welding standard operating procedures for welding, strictly control the installation of the system according to the principle of 3D dead corners, and prevent residue from causing crystal corrosion;
Choose a reliable spray device to prevent the introduction of exogenous iron ions caused by falling iron filings and to avoid dry rotation and friction of the spray ball;
Ensure good acid pickling passivation effect and effectively generate passivation film, perform periodic maintenance passivation on the system, regenerate passivation film, and recommend passivation cycle of 1-3 years/time;
Select high-quality raw materials for system installation, systematically trace the material reports of stainless steel pipes and fittings, and ensure the quality and polishing degree of 316L material; Introduce fluid analysis technology or surface analysis technology for red rust, install online monitoring devices for red rust, establish a comprehensive risk assessment mechanism, and detect and clean it early.



